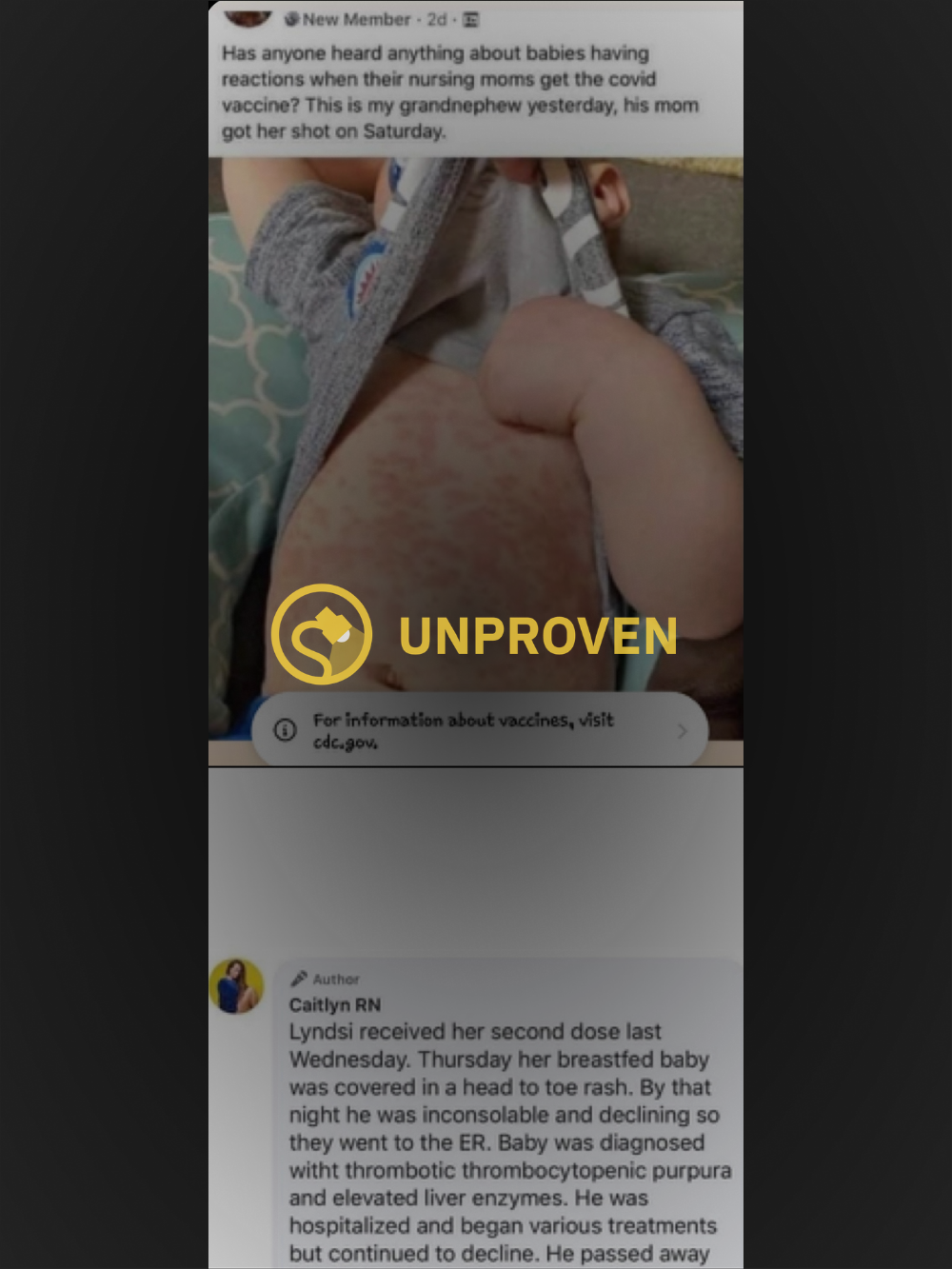
In late March 2021, claims of an infant dying after its breastfeeding mother received a second dose of an unspecified COVID-19 vaccine circulated on the internet, furthering a narrative that suggested vaccines may have understudied or unknown safety implications.
The above post includes what appears to be a photograph of the torso of an infant covered in a hives-like rash that was said to have occurred after its nursing parent received the “covid vaccine,” yet omits the name of the original poster and the group in which the message was posted. A Facebook user who goes by the name Caitlyn RN, and whose timeline revealed a number of posts that expressed opposition to vaccinations, commented on the post with the below story:
Lyndsi received her second dose last Wednesday. Thursday her breastfed baby was covered in a head to toe rash. By that night he was inconsolable and declining so they went to the ER. Baby was diagnosed witht thrombotic thrombocytopenic purpura and elevated liver enzymes. He was hospitalized and began various treatments but continued to decline. He passed away last night.
In mid-March, some websites and a number of social media users also posted the story in a chain-letter-style Facebook post with information copied and pasted rather than shared from the original post. Furthermore, the content of the post was vague and in no instance gave the last name of “Lyndsi," her location, or any other identifying characteristics. In most instances, users did not cite an original source but simply credited the story to an unnamed friend.
Our investigation determined that the photo in question likely depicts an authentic rash on a child, but we cannot say with certainty what type of rash it was or what might have caused it. Nor did the original poster include any identifying information that would allow our team to track a death certificate or any other information about the child or its condition. Thus, we rate this claim as “Unproven.”
Identifying the rash featured in the photograph is difficult, especially considering that rash-inducing viral infections are not uncommon in children and can be caused by a number of viruses, including chickenpox, measles, rubella, mononucleosis, and certain types of herpes infection. Though it is true that such rashes could result in elevated liver enzymes or a rare blood disorder known as thrombotic thrombocytopenic purpura, there is no evidence to suggest that any of the above conditions may be caused by vaccine fluid emitted via breastmilk.
To understand why such an occurrence is extremely unlikely, we must first understand how vaccines impact the human body. As of this writing, the U.S. Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUA) for the Pfizer-BioNtech mRNA for use in individuals age 16 years and older as a two-dose regimen given three weeks apart; the Moderna vaccine for use in those age 18 and older as a two-dose regimen given one month apart; and the Johnson & Johnson vaccine for use in individuals age 18 and older as a single dose regimen.
“These conversations are challenging because the Pfizer/BioNtech vaccine trial excluded lactating individuals. As a result, there are no clinical data regarding the safety of this vaccine in nursing mothers,” wrote the Academy of Breastfeeding Medicine in a statement. “However, there is little biological plausibility that the vaccine will cause harm, and antibodies to SARS-CoV-2 in milk may protect the breastfeeding child.”
This is largely due to the way in which a vaccine is produced and how it enters the body. Vaccines like Pfizer and Moderna are made of lipid nanoparticles that contain mRNA from the SARS-CoV-2 spike proteins. These particles are injected into the muscle, taken up by the muscle cells, and then transcribe to produce spike protein. It is this protein that stimulates an immune response and protects the individual against the virus.
First and foremost, it is unlikely that a vaccine lipid would enter the bloodstream and reach the breast tissue. Even if it did, it is less likely that either an intact nanoparticle or mRNA would be transferred into the breastmilk. And in the extremely unlikely event that mRNA was present in milk, it would be digested by the child — not entered into their bloodstream — and is unlikely to impose any biological side effects.
In fact, experts note that there could be an even bigger reward lurking in the breastmilk of vaccinated mothers. That’s because the antibodies and T-cells stimulated by the vaccine in the breastfeeding parent may transfer through breastmilk, in theory protecting the child from infection.
What the FDA Says
Snopes spoke with an FDA spokesperson who said that the agency takes all reports of adverse events related to vaccines seriously. In the case of COVID-19 vaccines, the FDA works closely with the Centers for Disease Control and Prevention (CDC) in the surveillance of vaccines being administered under an EUA in order to identify and address potential safety concerns. In addition, the FDA and CDC have a wide array of systems in place to monitor the safety of vaccines.
One such program is the Vaccine Adverse Event Reporting System (VAERS), a passive reporting system overseen by the CDC that receives unverified reports of adverse events following immunization with both licensed (approved) vaccines and the FDA-authorized COVID-19 vaccines. A search through the VAERS online database CDC Wonder for available data through March 19 did not reveal any record of an infant who had died as a result of their mother receiving the vaccination.
“Any reports of death following the administration of vaccines are promptly and rigorously investigated jointly by FDA and CDC. Such an investigation includes working with health care providers to obtain medical histories and clinical follow-up information,” noted the spokesperson.
“It is important to note that adverse events reported to VAERS following the administration of a COVID-19 vaccine do not necessarily indicate a causal relationship between receipt of the vaccine and the event.”
However, it is important to note that there is a lag time between reports to VAERS and when the reports are available online, and a subsequent look at the data revealed there was a corresponding case
On April 23, former New York Times reporter Alex Berenson shared a screenshot of a corresponding case that described a five-month-old infant who died of thrombotic thrombocytopenic purpura, a serious but rare blood clotting disorder. Berenson described the recording as an “authentic and highly detailed report.”
Our own search of case number 1166062-1 revealed that there was such a case filed in the VAERS system:
The report was filed on April 4 — four days after our initial reporting of this event. It described a five-month-old male infant who had a recorded vaccination date of March 17 with an onset of symptoms the following day. A description of the adverse event read:
Patient received second dose of Pfizer vaccine on March 17, 2020 while at work. March 18, 202 her 5 month old breastfed infant developed a rash and within 24 hours was inconsolable, refusing to eat, and developed a fever. Patient brought baby to local ER where assessments were performed, blood analysis revealed elevated liver enzymes. Infant was hospitalized but continued to decline and passed away. Diagnosis of TTP. No known allergies. No new exposures aside from the mother’s vaccination the previous day.
First and foremost, the above description listed a vaccine receipt date of 2020, not 2021. And as some Twitter users pointed out, while healthcare providers are required by law to report to VAERS, anyone can file a VAERS report. Just because a case is listed in the system does not mean that it is authentic or has been validated by medical professionals. (It is also important to note that knowingly filing a false VAERS report is a violation of Federal law (18 U.S. Code § 1001) punishable by fine and imprisonment.)
“VAERS is a passive reporting system, meaning it relies on individuals to send in reports of their experiences. Anyone can submit a report to VAERS, including parents and patients,” read the CDC website. "The Vaccine Adverse Event Reporting System (VAERS) accepts all reports, including reports of vaccination errors.”
Furthermore, the claim alleges that the mother was vaccinated, but the case report suggests that the infant had been vaccinated.
Breastfeeding recommendations established by the CDC further rote that with the exception of smallpox and yellow fever vaccines, neither inactivated nor live-virus vaccines administered to a lactating woman affect the safety of breastfeeding for women or their infants. Guidelines set forth by the American College of Obstetricians and Gynecologists (ACOG) recommended that COVID-19 vaccines be offered to both pregnant and lactating individuals.
According to the EUA factsheets provided to healthcare professionals for Pfizer-BioNtech, Moderna, and Janssen COVID-19 vaccines, available data on COVID-19 vaccine administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy because they have not been tested in pregnant women, limited data for use in pregnancy and clinical trials are ongoing, and pregnant or breastfeeding individuals should discuss options with their provider.
“ACOG recommends COVID-19 vaccines be offered to lactating individuals. While lactating individuals were not included in most clinical trials, COVID-19 vaccines should not be withheld from lactating individuals who otherwise meet criteria for vaccination. Theoretical concerns regarding the safety of vaccinating lactating individuals do not outweigh the potential benefits of receiving the vaccine. There is no need to avoid initiation or discontinue breastfeeding in patients who receive a COVID-19 vaccine,” wrote the organization in a March 24 statement.
While more data is needed to test the safety and efficacy of the vaccine in pregnant and lactating individuals, healthcare experts largely agree that the protection of serious disease provided by the vaccine outweighs any perceived risk that it may impose.