Key Facts:
- It is true that an ingredient found in Simparica TRIO, called sarolaner, has been associated with seizures, particularly in dogs with neurological disorders.
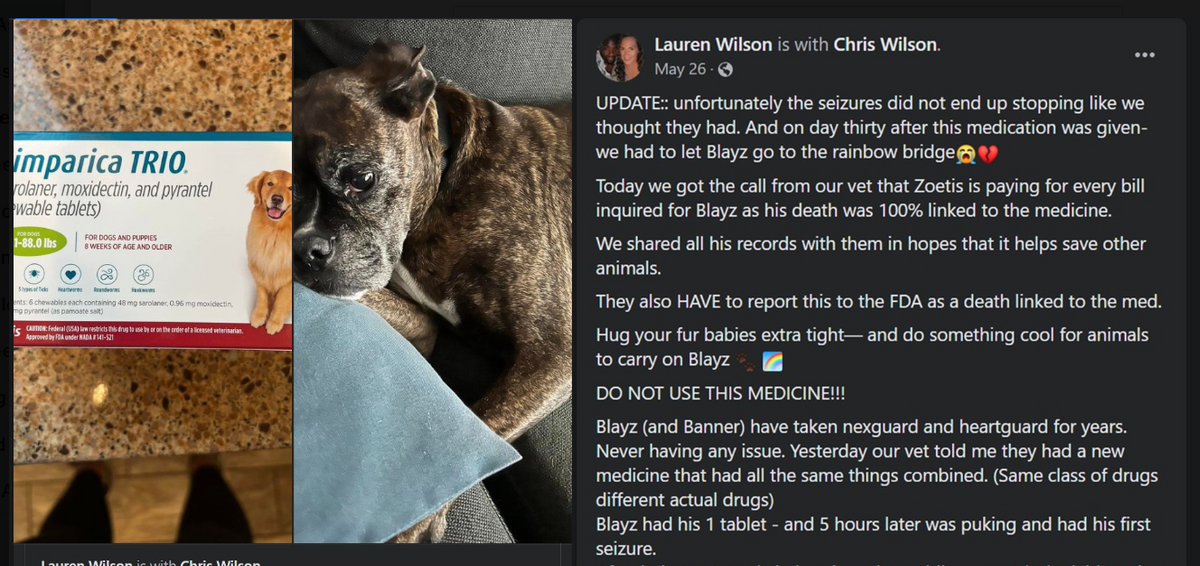
- Dog owner Lauren Wilson claims that Simparica's manufacturer, Zoetis, offered to pay for her veterinary expenses after her 10-year-old boxer died following his first and only dose of the drugs. An email from Zoetis confirmed the company was providing financial assistance to Wilson.
- However, Zoetis argues that because the dog was cremated before being necropsied, a definitive link between the drug administration and the dog’s death cannot be established.
A 10-year-old boxer with no history of neurological disorders or seizures reportedly died after being administered Simparica TRIO, an anti-parasitic drug meant to fend off fleas and ticks, as well as hookworms and roundworms.
Blayz had been given his first and only dose of the three-medication pill five hours earlier when, his owner, Lauren Wilson, told Snopes, he began puking and had his first seizure. At 4:30 a.m. the following morning, the boxer had a second seizure and was later taken to the vet.
Wilson described the incident in a May 26, 2022, Facebook post that has since been shared more than 271,000 times. (The full post can be read here and is archived here.)
For a month after receiving the single dose on May 26, Blayz suffered from seizures and finally had to be euthanized, Wilson said.
“He ended up having a horrible seizure [on] June 26 that led to him not being able to move other than lay there, fixed eyes. He was mostly gone — couldn’t even hold his head up — barely breathing. So, we euthanized, but we would have passed on likely that night. I couldn’t watch him suffer anymore though, at that point,” said Wilson.
Located in Iowa, Dr. Jamie Bunn at the Waukee Clive Veterinary did some blood work following Blayz’s seizures, Wilson said, and had told her that there was “no reason he should have been having them besides the meds.” Snopes was told that the vet had contacted the company and was passing along records to report the dog’s death. Wilson said that she was expecting a check in the mail, and Zoetis, the manufacturer of Simparica TRIO, confirmed that it was providing financial assistance. Wilson told our newsroom that she will send us the medical file of her dog with the bloodwork included, and we we will add that information to this article once we have received it. We have also reached out to Bunn for further verification regarding the incident.
Simparica TRIO is a chewable tablet that contains three main ingredients: sarolaner, moxidectin, and pyrantel. Sarolaner is associated with adverse reactions in dogs with a history of seizures or neurological disorders (more on that later), neither of which Blayz was known to have. Simparica TRIO was approved by the U.S. Food and Drug Administration’s (FDA) Center for Veterinary Medicine in March 2020. Sarolaner had been approved in 2016 to treat flea and ticks, moxidectin was approved in 1997 to prevent heartworm, and pyrantel was approved in 1977 to treat roundworms and hookworms. This marked the first time that the trifecta had been approved in a single combination pill. The prescription-only chewable tablet is given once a month to dogs and puppies eight weeks and older.
Side effects include: vomiting, diarrhea, lethargy, anorexia, otitis externa (ear infection), pruritus (itching), polyuria (urinating more frequently), hyperactivity, and polydipsia (drinking more water).
In clinical studies (described here), neurological side effects were not observed. However, sarolaner is a drug in the isooxazoline drug class. The FDA generally considers these to be safe and effective, but they come with a public alert issued in August 2021 that described, in some cases, an association with neurological adverse reactions, including “muscle tremors, ataxia, and seizures in some dogs and cats.”
A safety warning accompanied the 2020 news release announcing the approval of Simparica TRIO. It read:
Use with caution in dogs with a history of seizures. Simparica Trio contains sarolaner, a member of the isoxazoline class, which has been associated with neurologic adverse reactions including tremors, ataxia, and seizures in dogs with or without a history of neurologic disorders. The safe use of Simparica Trio has not been evaluated in breeding, pregnant, or lactating dogs. The most frequently reported adverse reactions in clinical trials were vomiting and diarrhea. See full Prescribing Information.
The prescribing information pamphlet also cautioned against the use of Simparica TRIO in dogs with a history of seizures or neurological disorders:
Sarolaner, one of the ingredients in SIMPARICA TRIO, is a member of the isoxazoline class. This class has been associated with neurologic adverse reactions including tremors, ataxia, and seizures. Seizures have been reported in dogs receiving isoxazoline class drugs, even in dogs without a history of seizures. Use with caution in dogs with a history of seizures or neurologic disorders.
Wilson told Snopes that Blayz did not have a history of either seizures or neurological disorders until after being given Simparica TRIO. We should note the possibility that her dog had an undiagnosed or underlying condition that was made worse by having been given the medication.
VCA Animal Hospitals explains that sarolaner is ingested by dogs and distributed through their body. When fleas or ticks bite the dog, they “are exposed to the drug and killed during their blood meal.” The medication lasts in the dog between four and six weeks. VCA adds, again, that “sarolaner should be used with caution in dogs with a history of seizures.”
Snopes contacted Zoetis and was told that the company is “aware of the social media post.” We specifically asked if administration of Simparica TRIO was deemed the cause of Blayz’s death and if the company had agreed to pay for the associated vet bills. We received the statement below:
At Zoetis, we are pet people, with many of our colleagues being pet owners themselves. Committed to providing safe and effective products to veterinarians, pet owners and the animals in their care, we empathize with the frustration and concern that fellow pet owners experience when their pets are sick.
Simparica Trio has been approved by the FDA as safe and effective. It is one of seven parasite prevention medications for dogs in the isoxazoline class currently on the market in the U.S. The FDA has published a fact-sheet reiterating that products in the isooxazoline class can and have been safely used in the majority of dogs. The fact-sheet, linked here also contains information for pet owners and veterinarians to consider when choosing flea and tick products for their pets. The product label and all related promotional materials for Simparica Trio have always included information about neurologic signs such as tremors, unsteadiness, and/or seizures that have been associated with use of medicines in the isoxazoline class in some dogs.
We are confident that Simparica Trio, which has been prescribed to protect over 10 million dogs since coming to the U.S. market in 2020, remains an effective and safe parasite preventative option for most dogs. The overall global reporting rate for any clinical sign reported for Simparica Trio (including vomiting, lethargy, diarrhea, or any neurological sign) is classified as “very rare”, which is defined by international regulatory authorities as less than 1 report per 10,000 doses administered. Simparica Trio’s adverse event profile continues to be predictable and consistent with pre-approval studies and looks similar to other isoxazoline products on the market.
Our team of veterinarians and veterinary technicians offer a variety of resources to pet owners. While we would not comment on interactions with individual pet owners, we do review cases and determine if financial assistance is warranted on a case-by-case basis. In this particular situation, no conclusive diagnosis was made as the dog was cremated, precluding a necropsy.
All adverse events reported to Zoetis are handled by our teams and reported to the FDA as part of our standard procedures and in compliance with U.S. regulations.
In short, the company stated that the cause of death cannot definitively be linked to the administration of Simparica TRIO. In a follow-up email to Snopes, the company confirmed that it was "providing financial support to Ms. Wilson."
We also reached out to the FDA for further information regarding reporting requirements but have not heard back as of this writing.
As for Wilson, she says she hopes her story will potentially prevent a similar situation for other dog owners and their furry friends.
“Sharing has already potentially saved some pups’ lives. Whether they [dogs] would have reacted poorly we won’t ever know, but I know if I knew the risks were there even for a neuro-typical dog, I would have never taken the risk,” she told Snopes.
If your dog or cat experiences an adverse reaction while using an isooxazoline product like Simparica, the FDA advises first contacting your veterinarian.
“The FDA continues to monitor adverse drug event reports for these products and encourages pet owners and veterinarians to report adverse drug events,” writes the agency. “You can do this by reporting to the drugs’ manufacturers, who are required to report this information to the FDA, or by submitting a report directly to the FDA.”
Suspected adverse reactions can be reported to Zoetis at +1- 888-963-8471. Direct reports to the FDA can be submitted here. Those with additional questions can contact the FDA at AskCVM@fda.hhs.gov or call 1-240-402-7002.