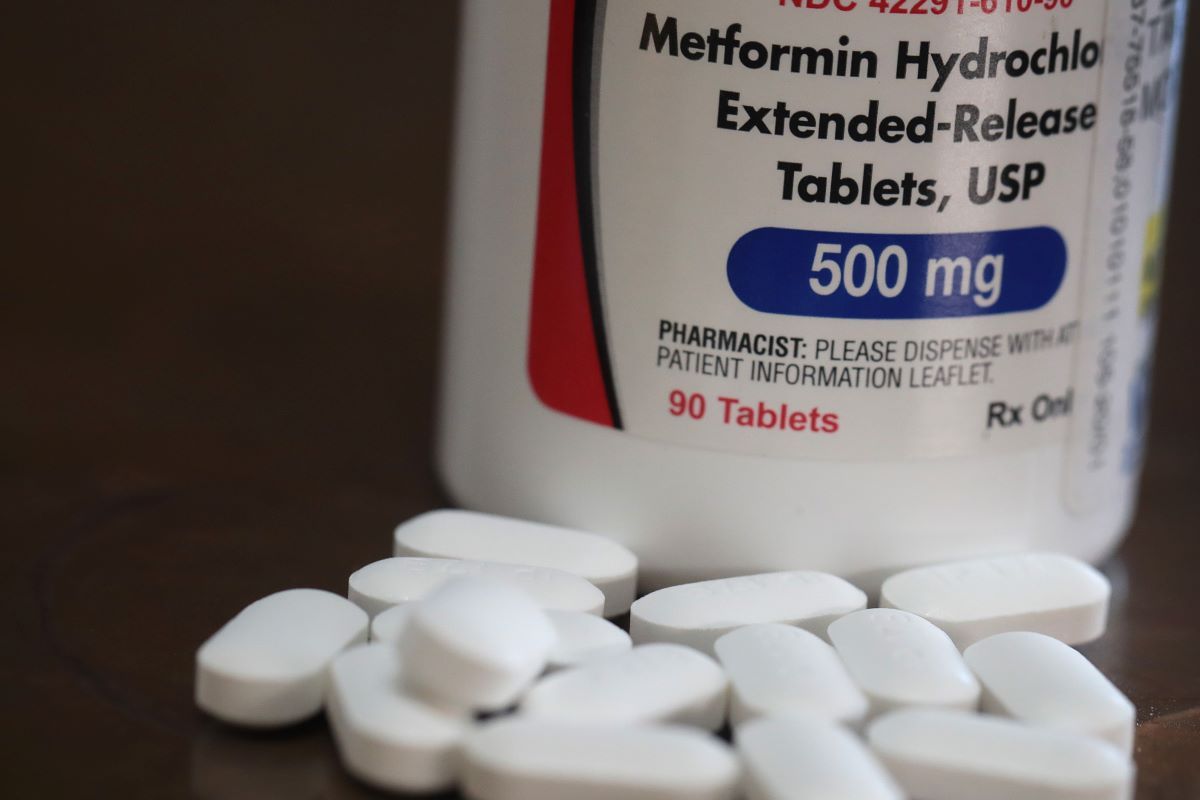
Some laboratories voluntarily recalled certain lots of the prescription drug Metformin Extended Release (ER) because it may contain N-nitrosodimethylamine (NDMA) above an acceptable intake limit. According to the Food and Drug Administration (FDA), "NDMA may increase the risk of cancer if people are exposed to it above the acceptable level and over a long period of time."
The FDA did not find NDMA in Immediate Release (IR) Metformin products (the most commonly prescribed type of metformin). This does not mean people should stop taking the drug. The FDA recommends that people prescribed metformin should continue taking it until their doctor prescribes an alternative.
A voluntary recall of Metformin, a prescription medication for type 2 diabetes patients, has caused alarm among some Snopes readers, with many asking if the drug could cause cancer.
We learned that through 2020 and early 2021, a number of pharmaceutical companies and laboratories voluntarily recalled the Metformin drug that was set for extended release (ER), due to the possibility that they contained the N-nitrosodimethylamine (NDMA) compound above an acceptable limit. If people are exposed to this particular compound above that acceptable limit over a long period of time, it could increase the risk of cancer.
But this should not be cause for panic according to the U.S. Food and Drug Administration (FDA).
According to the latest FDA update on the situation in January 2021:
Patients taking recalled ER metformin should continue taking it until a doctor or pharmacist gives them a replacement or a different treatment option. It could be dangerous for patients with type 2 diabetes to stop taking their metformin without first talking to their health care professional. FDA recommends that health care professionals continue to prescribe metformin when clinically appropriate.
FDA testing did not find NDMA in immediate release (IR) metformin, which according to the FDA is the most commonly prescribed drug.
The FDA published a list of the recalled drugs here.
According to the FDA, NDMA is classified as a “probable” human carcinogen and is a known environmental contaminant that is also found in water and foods, including meats, dairy products, and vegetables.
If you are a type 2 diabetes patient and your drug is on the recall list, the FDA recommends you continue taking it until consulting with a doctor. At the moment, the recalls are due to the possibility of higher levels of NDMA in ER metformin. Consult your health care provider about your specific case.
We thus rate this claim a “Mixture.”