Generally speaking, the meme repeats carefully selected, publicly known facts about the companies that developed COVID-19 vaccines with rough accuracy. However ...
These facts were cherry-picked and presented in a manner designed to foster the impression that the companies' COVID-19 vaccines aren't safe. But the meme neither demonstrates how the facts are relevant to the safety of the vaccines nor accounts for the scientific studies conducted thus far showing the vaccines to be overwhelmingly safe and effective.
As COVID-19 vaccines became widely available to Americans in spring 2021, anti-vaccine groups attempted to frame the shots' manufacturers as untrustworthy to try to stop people from getting inoculated.
Below is a sample of such claims — all from a single meme — that we fact-checked using reputable sources such as court records, resources distributed by the FDA and Centers for Disease Control and Prevention (CDC), and the vaccine manufacturers' websites.
All in all, the meme stated facts about companies that have developed COVID-19 vaccines but failed to demonstrate how those histories are relevant to the safety or effectiveness of the manufacturers' solutions for ending the pandemic.
Claim: Pfizer's '$4.7 Billion In Fines'
Firstly, in an attempt to persuade people against the two-dose Pfizer COVID-19 vaccine, the meme alleged the New York-based company faced almost $5 billion in penalties for supposedly breaking laws while manufacturing or distributing unidentified products.
"Pfizer: $4.7 billion in fines for false claims, drug and medical equipment safety violations, off-label promotion, corrupt practices, kickbacks, and bribery," according to the meme.
The claim was factually accurate. But its implication — that, because of prior lawsuits, Pfizer's COVID-19 vaccines were potentially unsafe — was unsubstantiated, a conclusion on which we elaborate below.
As one of the world's largest pharmaceutical corporations, Pfizer's multiple subsidiaries (which produce a range of drugs, including Advil, the erectile disfunction drug Viagra, and the anti-cholesterol drug Lipitor) indeed faced penalties totaling about $4.7 billion over the years, according to Good Jobs First, a left-leaning watchdog group tracking corporate subsidies.
Those cases, which originated in jurisdictions nationwide, essentially included the above-listed offenses involving all sorts of products between 2000 and 2019, according to Good Jobs First's database.
For instance, in 2004, the Warner-Lambert company — which Pfizer acquired four years earlier — pleaded guilty to illegally marketing the epilepsy drug Neurontin "even when scientific studies had shown it was not effective," the Department of Justice (DOJ) said in a statement.
Pfizer agreed to pay $430 million, said that it "cooperated fully with the government to resolve this matter," and stressed the alleged violations occurred before Pfizer acquired Warner-Lambert.
Then, in 2009, the company paid the largest settlement for health care fraud to date, totaling $2.3 billion, according to the DOJ.
In that case, the company's subsidy Pharmacia & Upjohn Company pleaded guilty to promoting a painkiller Bextra "for several uses and dosages that the FDA specifically declined to approve due to safety concerns," and paid a $1.3 billion criminal fine, the DOJ said in a statement. Bextra had been taken off the market four years earlier.
Additionally, Pfizer paid $1 billion to resolve civil claims regarding not only Bextra but also the antipsychotic Geodon, the antibiotic Zyvox, and the anti-epileptic drug Lyrica, per the statement. Pfizer denied all of those accusations, aside from acknowledging the improper promotion of Zyvox, Reuters reported at the time.
Shortly after reaching the historic settlement, the company's general counsel told reporters that it regretted "certain actions in the past," but was proud of the action it had taken to strengthen its internal oversight.
But here's how the meme misled people: It lacked critical evidence to show how those cases against Pfizer were relevant to COVID-19 vaccines, pictured below.
It also failed to acknowledge that clinical trials have shown the vaccines to be safe and effective. In December 2020, the FDA issued what's called an "Emergency Use Authorization" that deemed a COVID-19 vaccine created by Pfizer and BioNTech, as well as one manufactured by competitor Moderna, safe and effective enough for mass production.
The CDC's advisory committee on immunizations also recommended the Pfizer-BioNTech vaccine, along with Moderna and Johnson & Johnson's (J&J) formulas. (Here's the CDC's explanation for how vaccines like Pfizer's — which uses mRNA technology — attempt to train people's immune systems into producing antibodies that can fight the coronavirus, if necessary.)
Early on, clinical trials showed the Pfizer vaccine 95% effective in preventing COVID-19. The company confirmed "high efficacy and no serious safety concerns" after a March 2021 follow-up study.
Because of those results, the FDA expanded Pfizer's eligible vaccine population to include adolescents between the ages of 12 to 15 in mid-May.
Also, at that time, Pfizer and Moderna were seeking the FDA's full, regular authorization for their inoculation formulas. That status, which requires at least six months of patient data, would allow the companies to begin marketing the shots.
In other words, if or when the FDA grants the Pfizer vaccine full approval, the company "can advertise on TV and promote their products under the watchful eye of the FDA," former FDA commissioner Dr. Robert Califf told CNBC.
To conclude our research, we reached out to Pfizer's communication's department with the following questions:
- Has any person or entity accused Pfizer of making false claims or illegally promoting COVID-19 vaccines, especially considering the fact the FDA hasn't yet granted the company the green light to promote the product?
- If so, what's the company's response to those allegations?
- What's Pfizer's response to people who are wary of the company's COVID-19 vaccine because of previous lawsuits in which Pfizer pleaded guilty to fraud?
No one has answered our inquiry, but we will update this report when, or if, that changes.
In sum, we rate the claim regarding Pfizer a "Mixture" of true and misleading information. It was an accurate depiction of the company's alleged violations involving a handful of its numerous drugs over years. However, no evidence connected those offenses with COVID-19 vaccines nor showed Pfizer had made false claims about, or illegally promoted, its vaccine.
Claim: Moderna 'Has Never Brought a Vaccine to Market'
Next, in attempt to deny the legitimacy of the Moderna COVID-19 vaccine, the meme claimed the Massachusetts-based company had tried numerous times to develop vaccines for mass production prior to the pandemic with no success.
"Moderna: Has never brought a vaccine to market since its founding, despite fielding 9+ vaccine candidates, none of which made it through phase 3 clinical trials," the meme alleged.
Similarly to the Pfizer allegation, the claim was rooted in truth. But the statement failed to explain how it was relevant to Moderna's COVID-19 vaccine, as well as erroneously implied the company's other vaccines did not reach mass distribution solely because of shortcomings in the products themselves, such as their effect on patients or alleged lack of success preventing viral outbreaks.
Rather, other barriers — such as a lack of funding for research — also played a role in the pharmaceutical company's vaccine history.
In addition to the COVID-19 vaccine, Moderna has developed nine prophylactic immunizations using mRNA technology since its founding in 2010, according to the company's website. The company describes mRNA "like software for the cell" with the potential to fighting many diseases.
In April 2020, before the FDA granted emergency authorization of Moderna's COVID-19 vaccine, BioSpace reported:
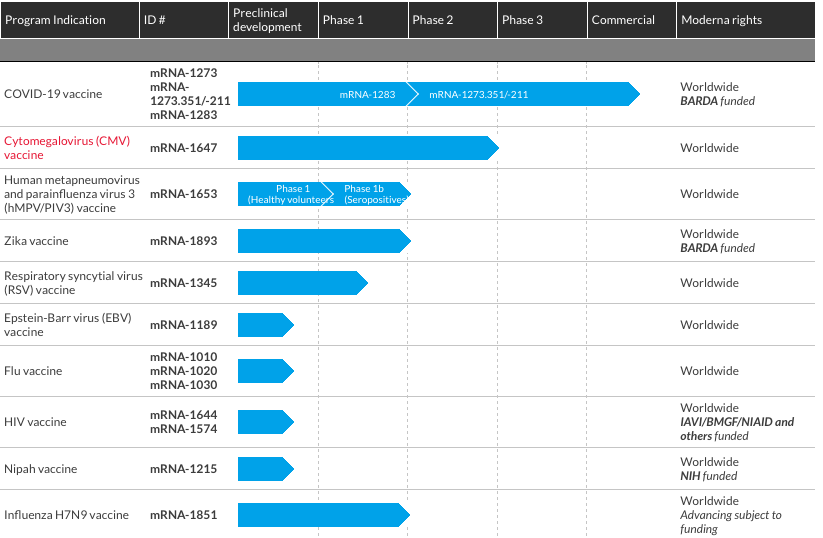
So far, Moderna has conducted early trials with success on nine vaccine candidates, including respiratory syncytial virus (RSV), human metapneumovirus (hMPV) and parainfluenza virus (PIV3), influenza H7N9, cytomegalovirus (CMV), Zika, Epstein-Barr, chikungunya. [...]
However, the meme was accurate in claiming none of those experimental immunizations (aside from the COVID-19 vaccine) progressed past Phase II clinical trails. That's the stage after researchers test a product on an initial group of people and want to see how it affects a larger sample. (See the FDA's website for more differences between phases of clinical research, and see here for Moderna's process for developing and testing its mRNA vaccines.)
Here's Moderna's visualization of its various vaccines and their development:
But, as you can see, Moderna's Influenza H7N9 vaccine was only advancing "subject to funding," negating the meme's implication that the company halted development solely due to safety concerns or ineffectiveness.
Furthermore, Moderna's Cytomegalovirus (CMV) vaccine was the first of its kind to enter a Phase II clinical trial, showed "promising" results so far, and was scheduled to progress in 2021, The Medicine Maker reported. Studies on the company's other immunizations remained ongoing, too.
Similar to the Pfizer COVID-19 vaccine, the Moderna shot was 94% effective in preventing recipients from catching the virus.
Lastly, Snopes contacted representatives of Moderna with the following questions:
- In summary, why have Moderna's nine experimental vaccines stalled in research and development?
- When does the company expect to advance one of its immunization formulas (aside from the COVID-19 vaccines) to the next stage?
- What's Moderna's response to people who are nervous about getting its COVID-19 shot because it's the company's first vaccination formula to reach commercial production?
Pending answers to those questions, we will update this report.
In short, the meme factually stated that the COVID-19 vaccine was Moderna's first inoculation to reach mass production. However, it simultaneously implied, without evidence to substantiate the claim, that the company's other vaccines stalled due to their effects on test patients. For those reasons, we rate the claim a "Mixture" of true and misleading information.
Claim: Johnson & Johnson 'Named in Hundreds of Thousands of Lawsuits'
Much like the meme's claim about Pfizer, its allegation that Johnson & Johnson was "named in hundreds of thousands of lawsuits for toxic and/or dangerous products, including drugs, shampoos, medical equipment, and asbestos-contaminated baby powder" was at least partially accurate at face value.
But the post failed to provide substantial evidence to prove how, or to what extent, that fact was relevant to the safety of the company's COVID-19 vaccine.
Let us explain. In fall 2020, Johnson & Johnson indeed paid over $100 million to resolve more than 1,000 lawsuits claiming that the pharmaceutical giant's banned powder and talc products caused cancer due to asbestos contamination, Bloomberg reported. Some 20,000 pending cases made similar accusations.
Additionally, unrelated lawsuits alleged the company violated medical equipment or drug safety guidelines, among other offenses, according to Good Jobs First, the corporate accountability watchdog. For instance, one investigation concluded the company did not fully disclose the risks of devices to support women's prolapsed pelvic organs, according to The Gaurdian.
Let us note here: The exact number of lawsuits that named the company for allegedly selling "toxic and/or dangerous products" since its founding was unknown, which meant the meme's reference to "hundreds of thousands" of cases was unsubstantiated.
No violation listed in the Good Jobs First database was related to Johnson & Johnson's single-dose COVID-19 vaccine, which the FDA approved months after Pfizer's and Moderna's, in early 2021.
Instead of using mRNA technology, the J&J shot takes the form of what’s called a viral vector to attack one specific part of SARS-CoV-2. AstraZeneca's COVID-19 immunization (which the FDA has not approved, as of this writing, and discuss below) uses that same process. The J&J shot was about 66% effective in preventing COVID-19 from infecting recipients during clinical trials, according to the CDC.
That said, J&J's immunization was not without controversy. In mid-April, mass vaccination sites in states including Georgia, Colorado, and North Carolina temporarily halted the shot's distribution after a few recipients felt dizzy, light-headed, and faint. The CDC monitored the reports and continued to recommend the shot's use.
Then, shortly later, the CDC recommended all vaccine providers nationwide to temporarily halt giving out the J&J shot while health officials investigated a potential blood clotting issue that occurred in seven cases out of 6.8 million shots administered.
That pause ended on April 23, when the CDC said in a statement:
- The FDA and CDC have confidence that this vaccine is safe and effective in preventing COVID-19.
- The FDA has determined that the available data show that the vaccine’s known and potential benefits outweigh its known and potential risks in individuals 18 years of age and older.
- At this time, the available data suggest that the chance of [the blood clotting issue] occurring is very low, but the FDA and CDC will remain vigilant in continuing to investigate this risk.
We reached out to J&J with the following questions:
- Has any person or entity accused J&J's COVID-19 vaccine of containing toxic or dangerous ingredients?
- If so, what's the company's response to those allegations?
- What's J&J's response to people who do not want to get the company's COVID-19 immunization because of lawsuits alleging J&J's other products contained harmful ingredients such as asbestos, a known carcinogen?
We will update this report when, or if, the company responds.
In conclusion, we also rated the meme's statements about J&J a "Mixture" of true and misleading information. While it was a mostly factual representation of the company's alleged lawsuits over the years, nothing linked those cases with the company's COVID-19 immunization nor showed that the product included toxic or dangerous ingredients, like the meme implied.
Claim: AstraZeneca 'Suspended by Two Dozen European Countries'
Of all of the meme's claims, the assertion about AstraZeneca was the least misleading. It was a direct reference to the manufacturer's COVID-19 vaccine (which is not currently in use in the U.S. and was developed in partnership with Oxford University) instead of an out-of-context fact pertaining to other pharmaceutical products.
"AstraZeneca: Suspended by two dozen European countries due to severe, lethal adverse reactions, like blood clots," the post alleged.
Yes, in spring 2021, a number of European countries (Denmark, Norway, Iceland, Bulgaria, etc.) temporarily suspended rollouts of the AstraZeneca COVID-19 vaccine after reports of patients developing blood clots. Less than 40 cases of blood clots were reported out of the 17 million vaccine recipients, according to AstraZeneca.
Reputable sources, including Snopes, said "more than a dozen European countries" issued the suspension — not "two dozen," like the meme claimed.
Additionally, the meme's use of the phrase "due to" discredited its message. Rather, AstraZeneca, health officials, the World Health Organization (WHO) and government regulatory bodies all said there was no causal link between the vaccine formula and the patients' blood clots.
"In fact, nearly every country that issued a suspension acknowledged that it had no evidence the vaccine had caused the blood clots," NBC reported at the time. "Health experts have pointed out that the people most likely to currently be receiving COVID-19 vaccinations are also more likely to have other health problems, which could put them at higher risk for blood clots."
See our fact check into the matter here.
Since then, many European countries restarted their programs after the European Medicine Agency dubbed the AstraZeneca vaccine “safe and effective" despite the blood-clot reports. EMA said in a statement:
- the benefits of the vaccine in combating the still widespread threat of COVID-19 (which itself results in clotting problems and may be fatal) continue to outweigh the risk of side effects;
- the vaccine is not associated with an increase in the overall risk of blood clots (thromboembolic events) in those who receive it;
- there is no evidence of a problem related to specific batches of the vaccine or to particular manufacturing sites;
- however, the vaccine may be associated with very rare cases of blood clots associated with thrombocytopenia, i.e. low levels of blood platelets (elements in the blood that help it to clot) with or without bleeding, including rare cases of clots in the vessels draining blood from the brain (CVST).
In other words, we rate the meme's claim regarding AstraZeneca a "Mixture" of false and factual information. It was true that a number of European countries temporarily suspended the use of the manufacturer's COVID-19 vaccine after a handful of recipients reported blood clots.
But no evidence proved those thromboembolic issues were a direct, adverse affect of the vaccine (rather than unrelated medical issues), and several countries restarted their AstraZeneca vaccination programs since the brief halt.